Unveiling the Wonders of Supramolecular Chemistry
Supramolecular chemistry, a captivating realm within the broader field of chemistry, delves into the intricate interactions between molecules. Unlike traditional chemical bonds, which involve the sharing or transferring of electrons, supramolecular chemistry explores non-covalent interactions that give rise to complex, higher-order structures. This article explores the fascinating world of supramolecular chemistry, shedding light on its principles, applications, and potential for advancements in various scientific domains.
Principles of Supramolecular Chemistry:
At the heart of supramolecular chemistry lies the study of non-covalent interactions, including hydrogen bonding, π-π stacking, van der Waals forces, and host-guest interactions. These weak forces allow molecules to assemble and organize into larger, more complex structures reminiscent of nature’s intricate biological systems.
One of the critical concepts in supramolecular chemistry is molecular recognition, where molecules selectively bind to one another based on complementary shapes and functional groups. This phenomenon mimics the specific interactions observed in biological systems, such as enzyme-substrate interactions and DNA base pairing.
Applications in Nanotechnology:
Supramolecular chemistry plays a pivotal role in nanotechnology, offering a versatile toolkit for designing and constructing nanoscale materials and devices. Researchers harness these non-covalent interactions to create self-assembled nanostructures with unique properties. These nanostructures find applications in drug delivery, sensors, and even in the development of molecular machines.
Supramolecular Chemistry in Drug Delivery:
The ability to design supramolecular structures at the nanoscale opens new frontiers in drug delivery. Researchers can engineer carriers that self-assemble to encapsulate therapeutic molecules, providing targeted delivery and controlled release. This enhances the efficacy of treatments and reduces side effects by minimizing the impact on healthy tissues.
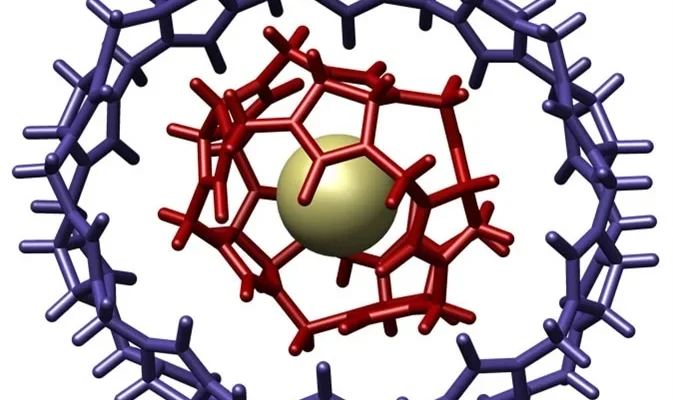
Host-Guest Chemistry and Material Design:
Supramolecular interactions, particularly host-guest chemistry, offer a compelling avenue for designing new materials with tailored properties. Molecular hosts, such as cyclodextrins, can encapsulate guest molecules, influencing their solubility, stability, and reactivity. This has implications for developing advanced materials, including responsive polymers and intelligent drug delivery systems.
Challenges and Future Prospects:
Despite its promising applications, supramolecular chemistry presents challenges, including the need for precise control over assembly processes and stability issues. Ongoing research aims to address these challenges and unlock the full potential of supramolecular systems in various scientific and technological domains.
The field of supramolecular chemistry continues to push the boundaries of what is possible at the molecular level. From mimicking nature’s precision to revolutionizing drug delivery and materials science, supramolecular chemistry is a testament to the creative power of non-covalent interactions. As researchers delve deeper into this fascinating world, the prospects for innovative applications and breakthroughs in science and technology appear boundless.
References:
- https://www.mdpi.com/journal/nanomaterials/special_issues/90Y32KE2QT
- https://pubs.rsc.org/en/content/articlepdf/2017/cs/c7cs00391a#:~:text=The%20modularity%20of%20supramolecular%20interactions,the%20precision%20of%20pharmaceutical%20practice.
- https://www.sciencedirect.com/topics/chemistry/supramolecular-chemistry
- https://en.m.wikipedia.org/wiki/Supramolecular_chemistry